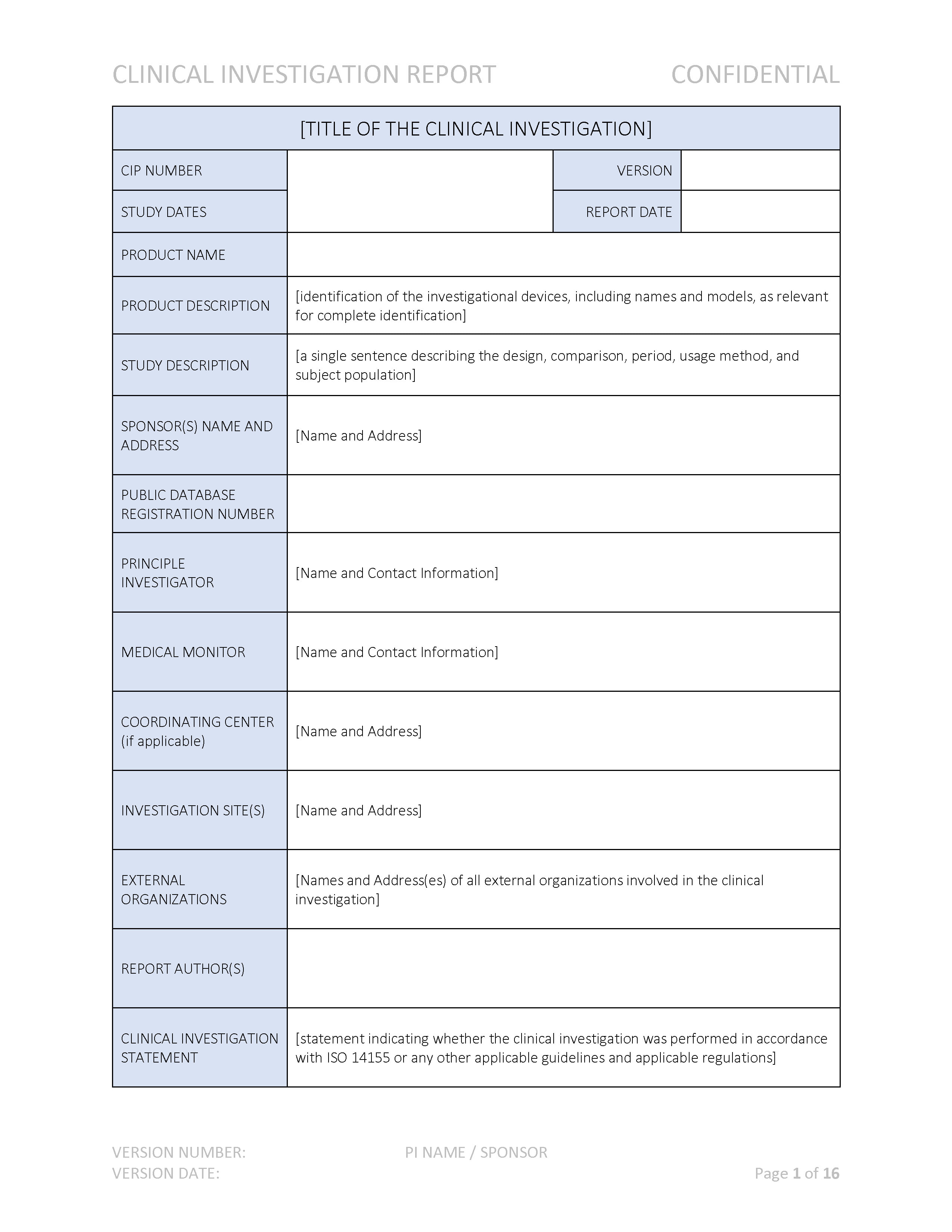
SOPs for GCP-Compliant Clinical Trials: A Customizable Manual for Sponsors of Medical Device Trials : MS Word Template | CenterWatch
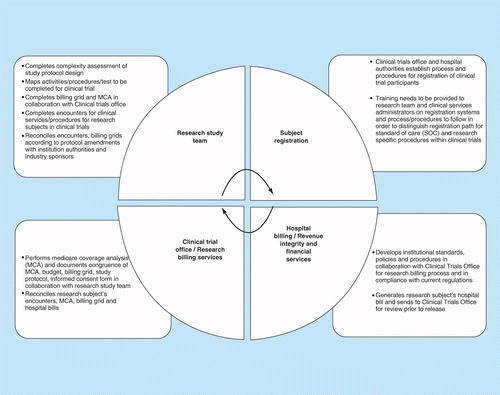
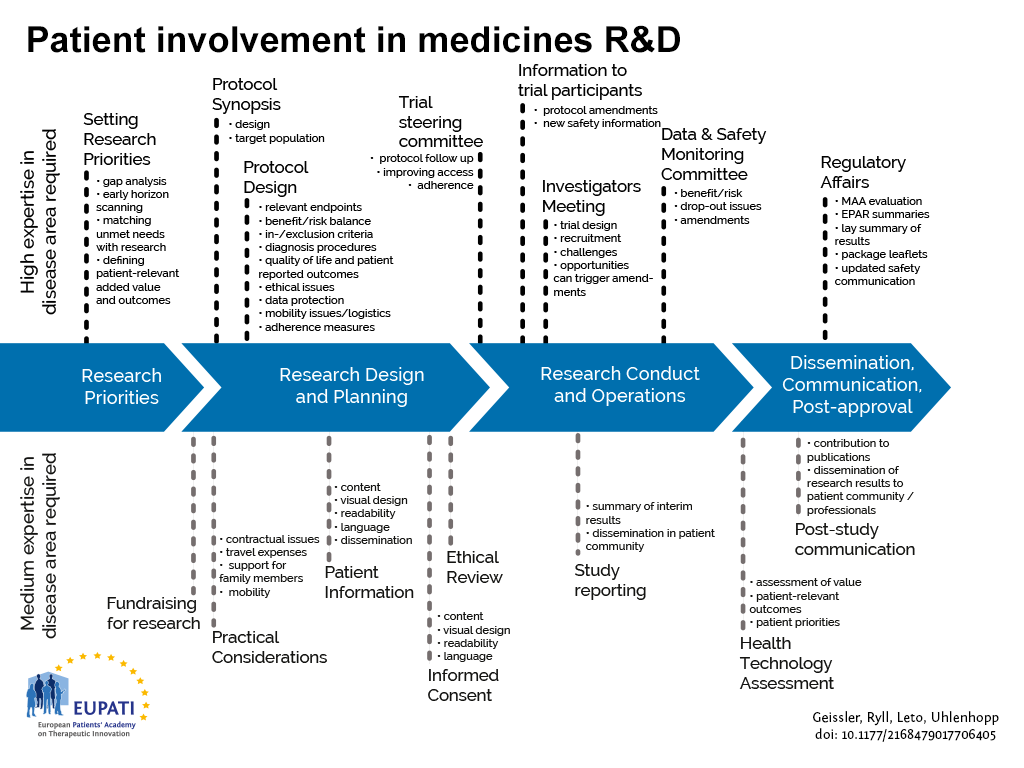
Optimization of protocol design: a path to efficient, lower cost clinical trial execution | Future Science OA