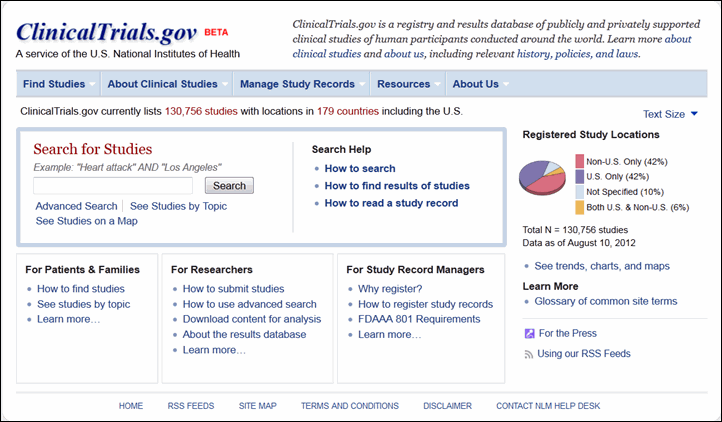
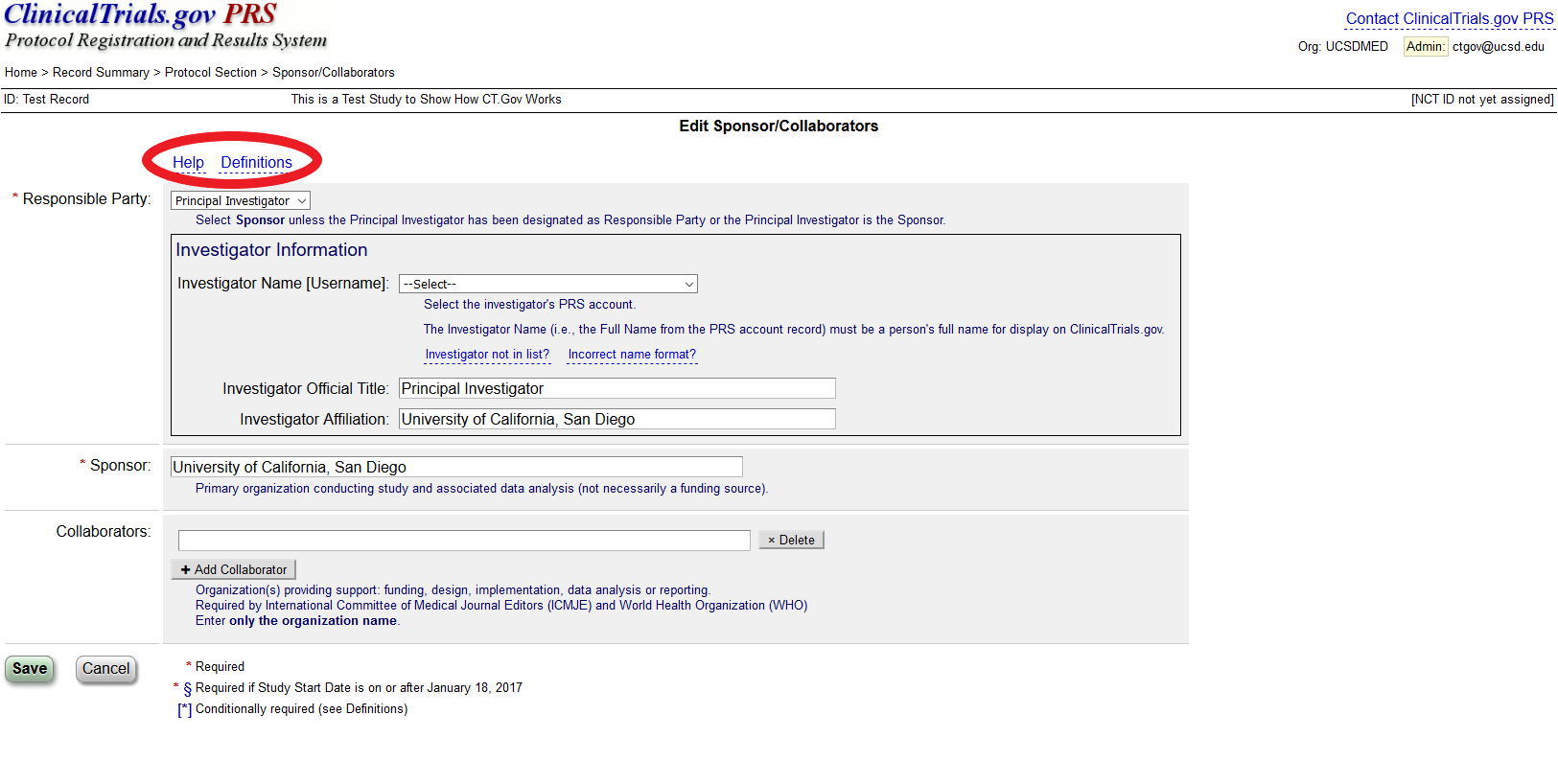
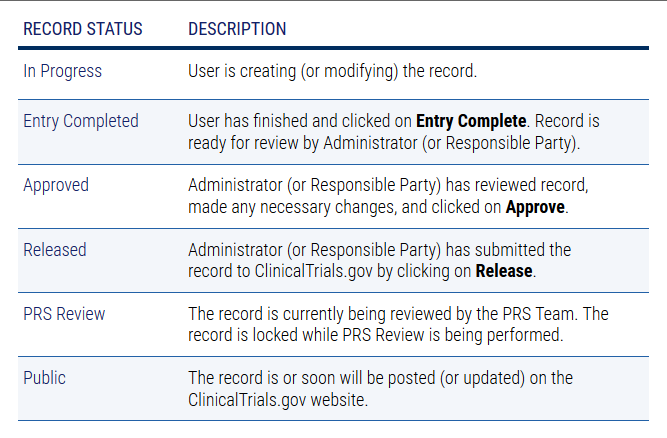
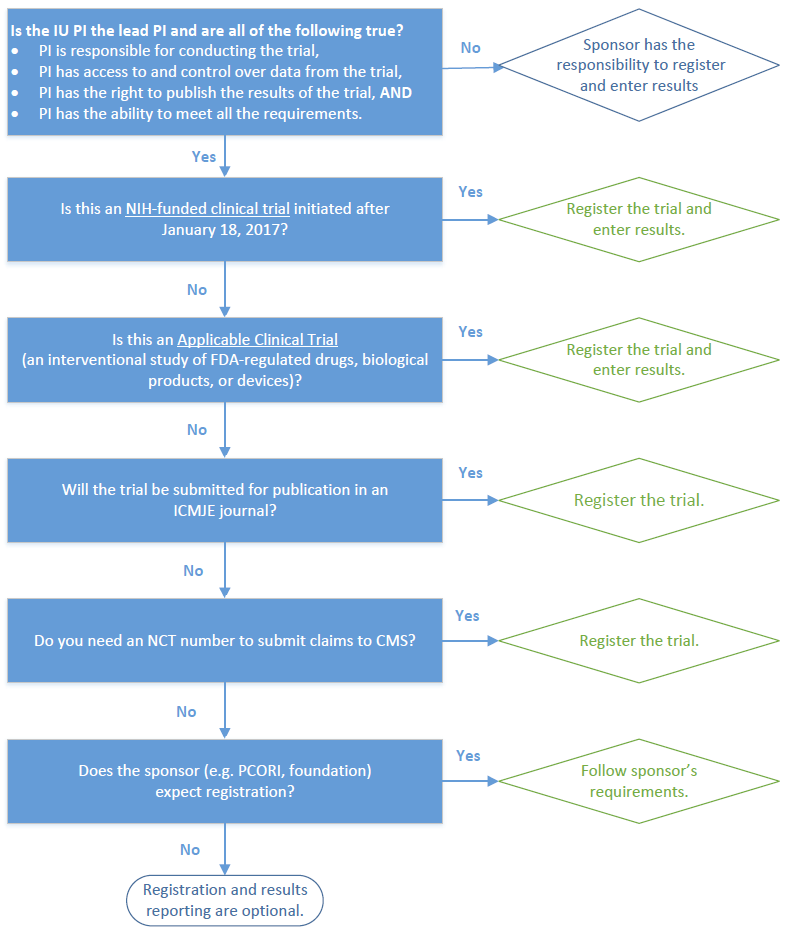
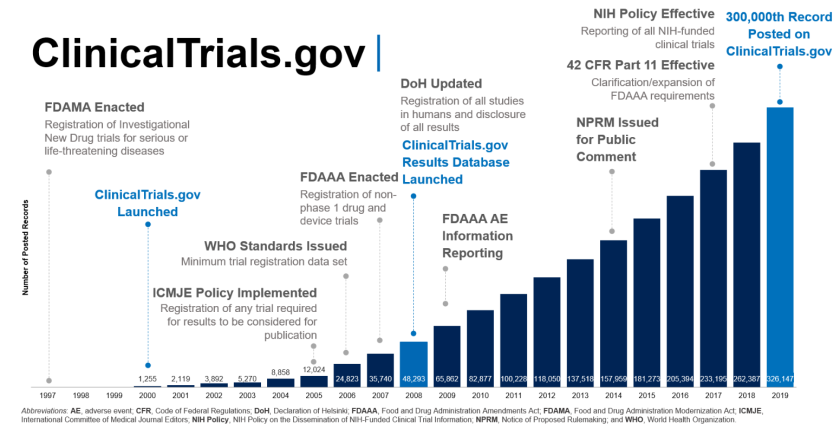
Celebrating 20 Years of ClinicalTrials.gov and Looking to the Future – NLM Musings from the Mezzanine
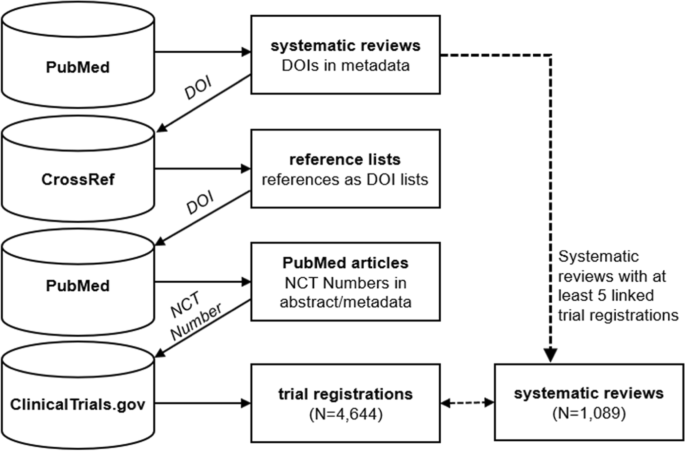
The automation of relevant trial registration screening for systematic review updates: an evaluation study on a large dataset of ClinicalTrials.gov registrations | BMC Medical Research Methodology | Full Text

NIH Issues Clinical Trial Transparency Proposal; Expands Submissions to Results From Unapproved Products, Requires Detailed Adverse Event Information – Policy & Medicine

Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis | The BMJ