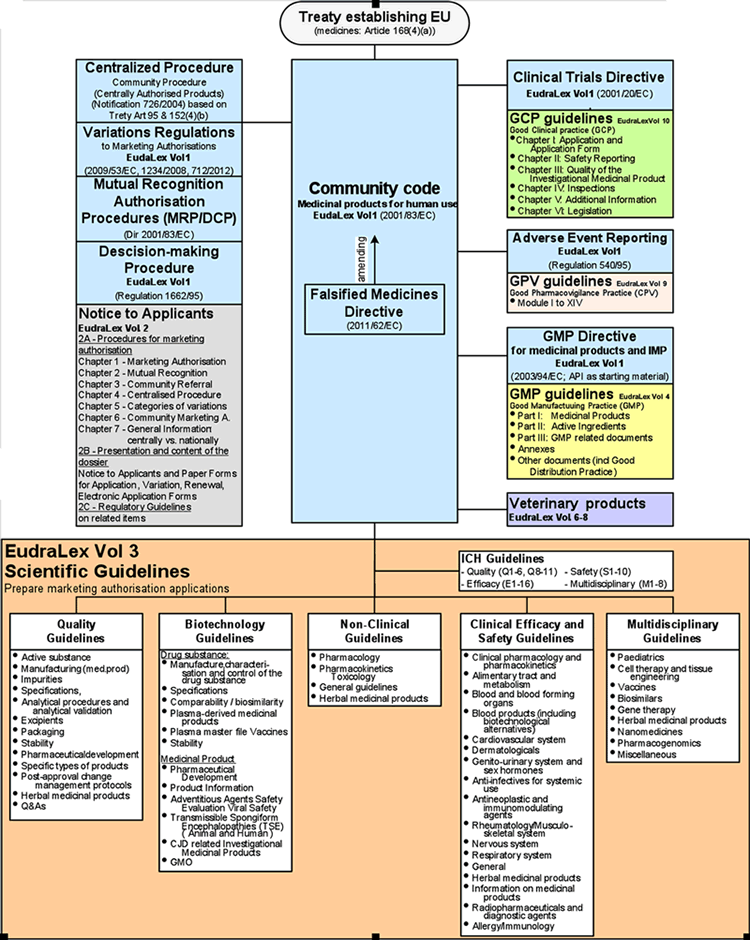
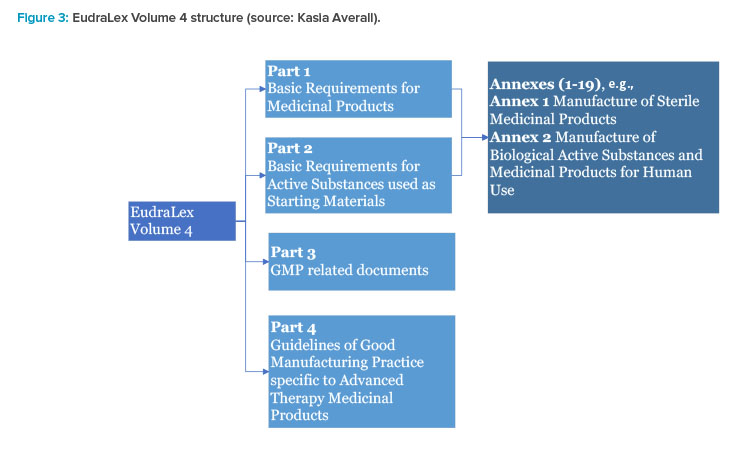
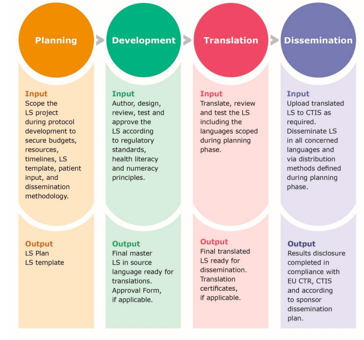
Principal Documents taken into account for the preparation of procedures for GCP inspections requested by the CHMP

EudraLex - Volume 10 Clinical trials guidelines and the impact of the new coming Regulation 536/2014

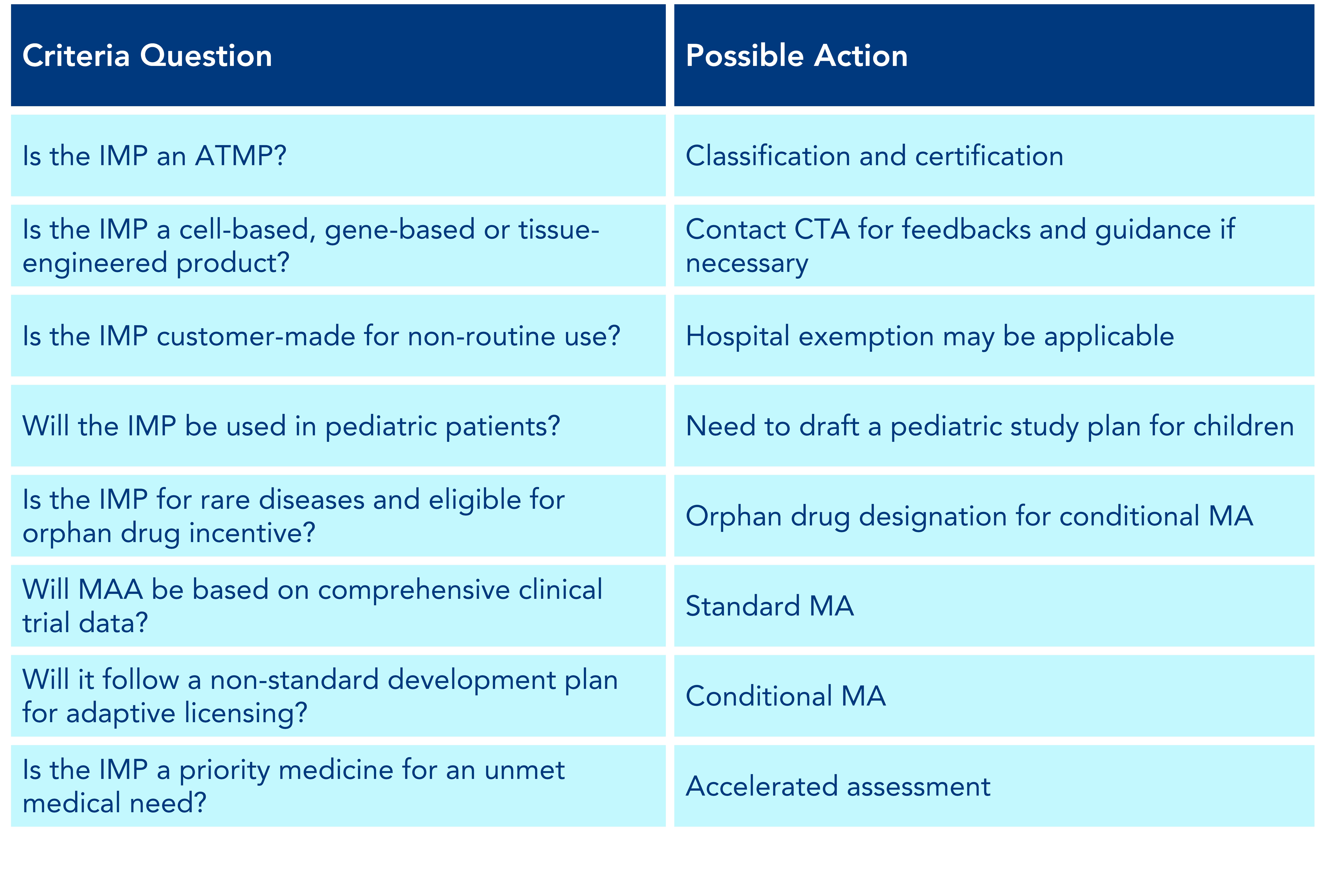
Frontiers | Advanced Therapy Medicinal Products' Translation in Europe: A Developers' Perspective | Medicine
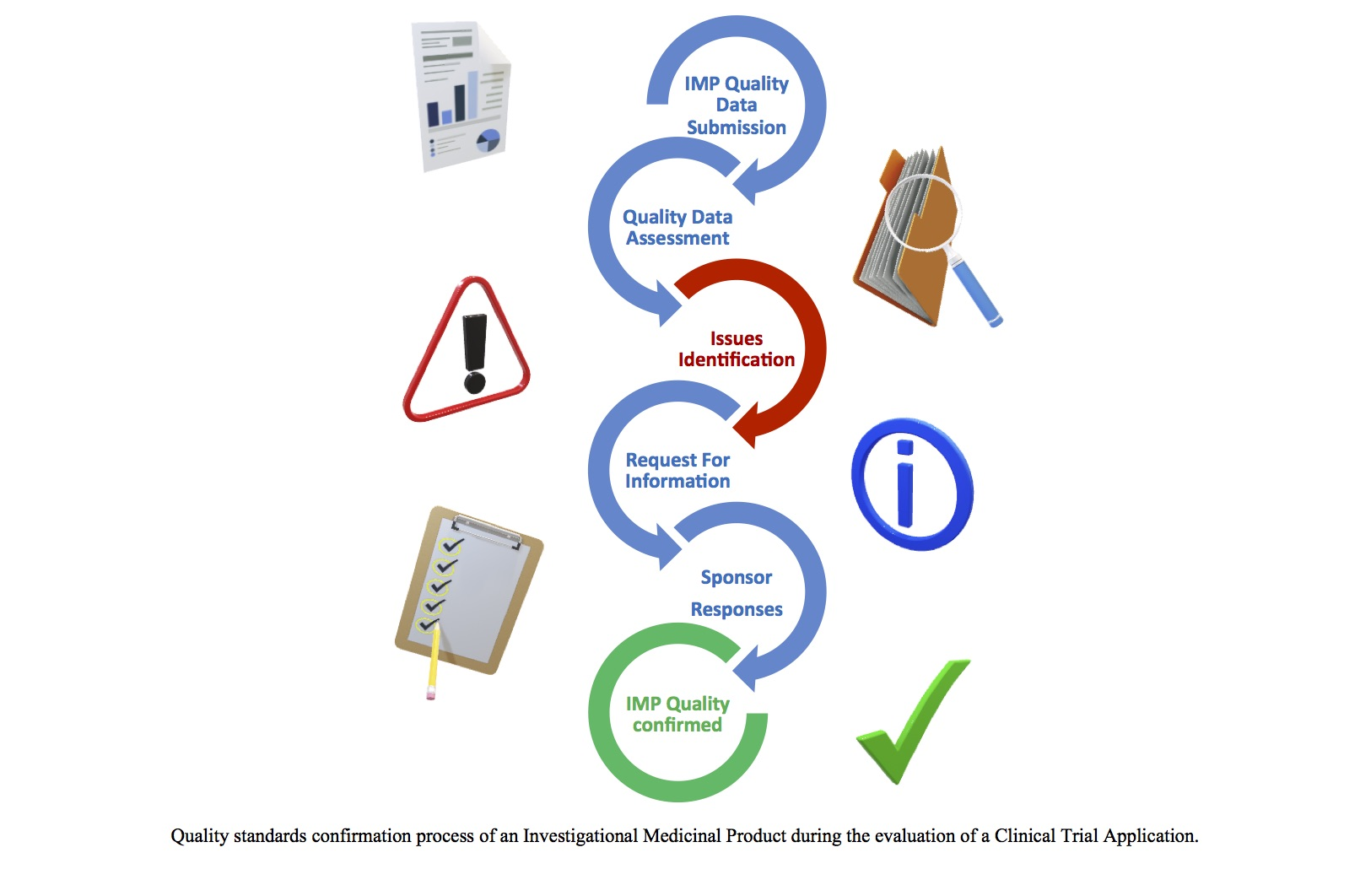
Pharmaceuticals | Free Full-Text | Quality Assessment of Investigational Medicinal Products in COVID-19 Clinical Trials: One Year of Activity at the Clinical Trials Office | HTML