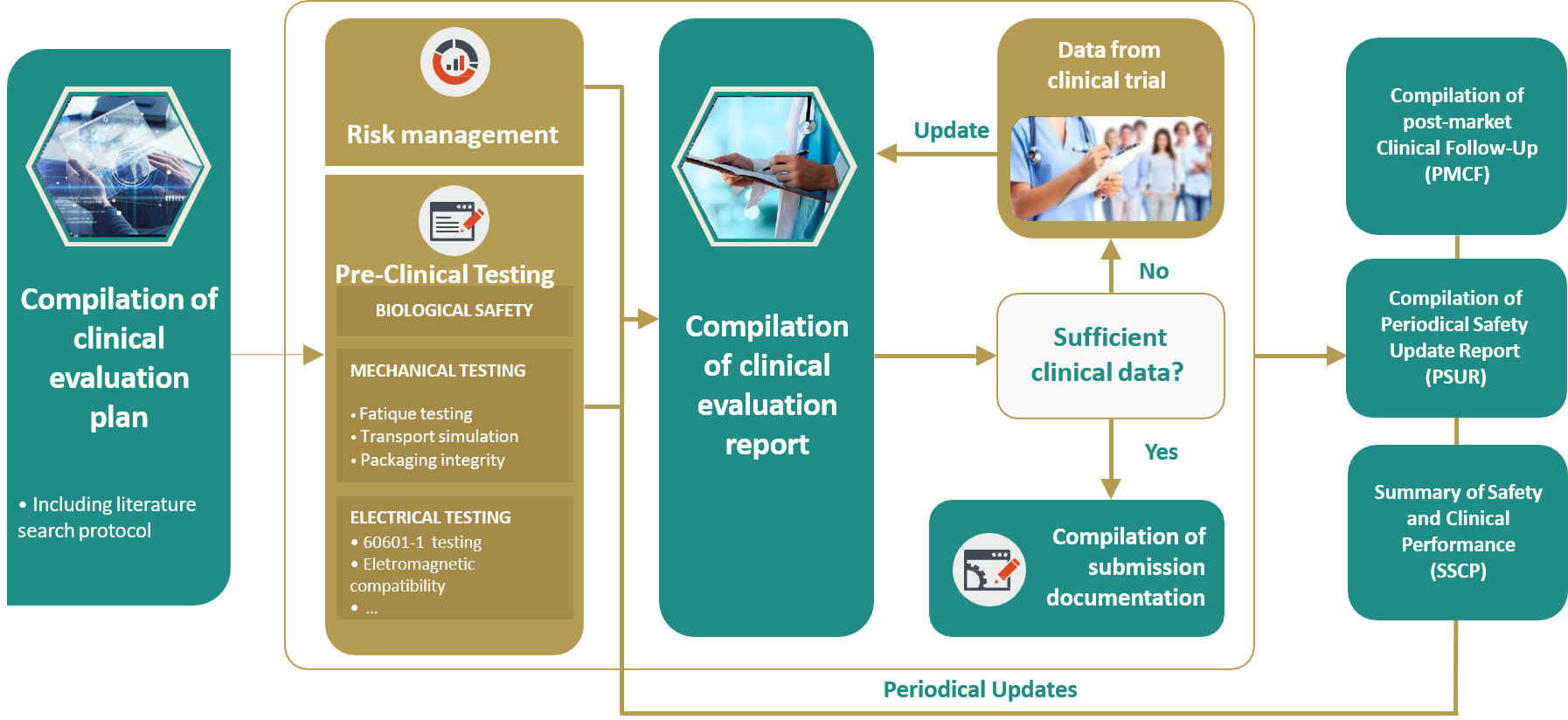
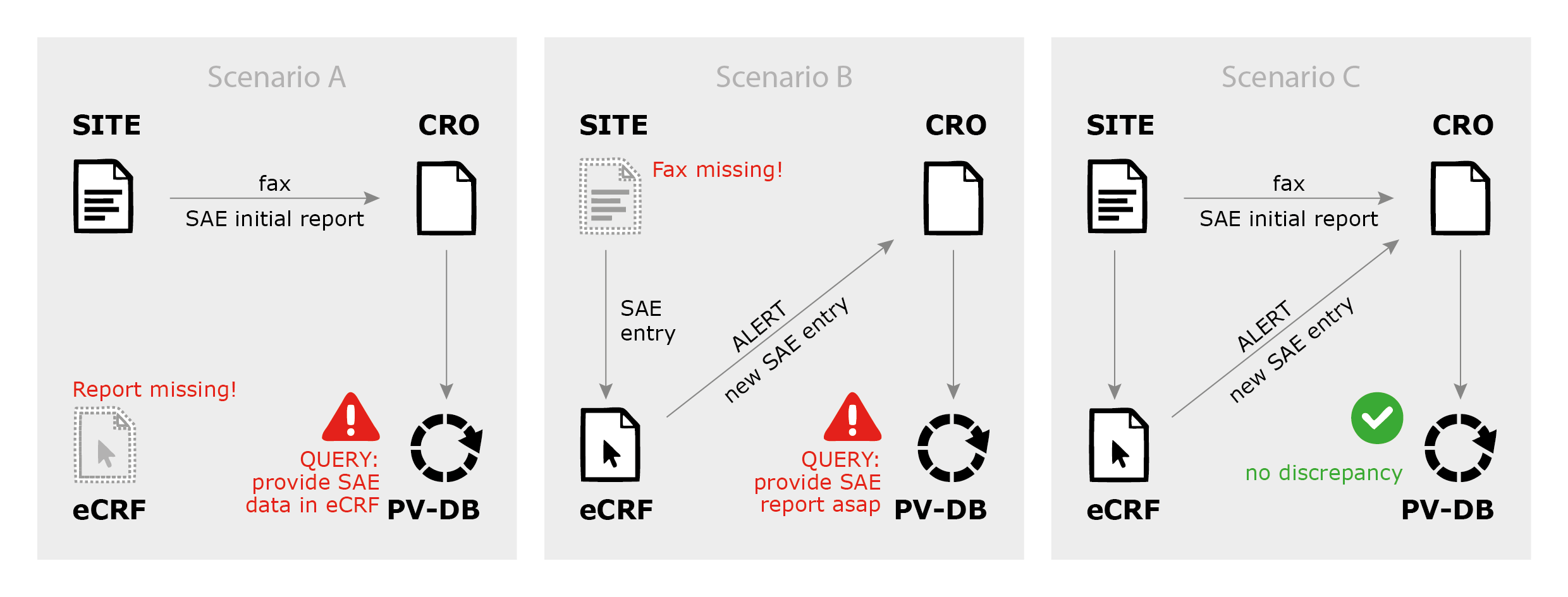
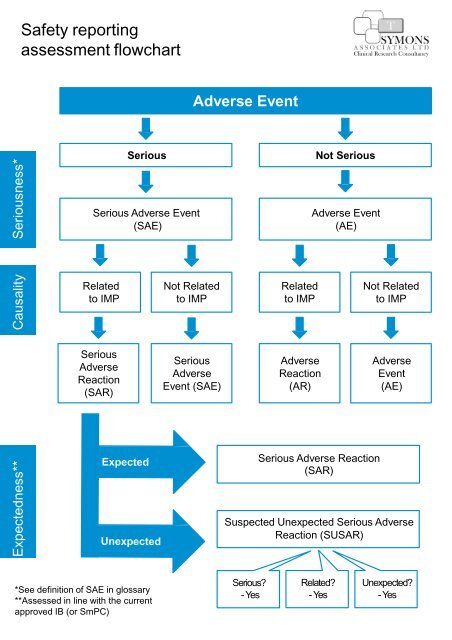
Safety Reporting Reference Model: Optimizing Global Safety Reporting in Clinical Trials using Local Laws - WCG Clinical

Simplifying safety reporting as part of clinical trial investigator retention - Drug Discovery and Development
Comparison between efficacy/safety and pragmatic trials. AE, adverse... | Download Scientific Diagram

Safety Reporting Overload in Clinical Trials: FDA and Site Perspectives on Overreporting of Adverse Events | CenterWatch